Water From Air
Extracting water from air takes energy, and there's no way to avoid that. So it makes the most sense to extract water from air using the most accessible and cheapest energy source. To the average person, these energy sources come from the sun and from the burning of biomass.Solar panels can produce electricity to run a dehumidifier type of device which condenses water out of the air. But you would need a lot of solar panels to produce enough electricity to get a significant rate of water production. This would be costly.
Alternatively, you can capture water from the air using a lot of Calcium Chloride until it becomes a liquid brine, and you can then evaporate it using a wood fire (or a fire from burning another biomass). This allows you to condense the resulting water vapor out of the air. You can then reuse the Calcium Chloride, which doesn't need to be returned to its original solid form in order for it to reabsorb water (but it might be easier to handle if it is solid).
Calcium Chloride has a very useful property in which it attracts water molecules from the surrounding environment. This is what makes it useful as a means of extracting water from the air. A picture of Calcium Chloride in its solid form is shown below.

Source: https://commons.wikimedia.org/wiki/File:Calcium_chloride_CaCl2.jpg. Author: Firetwister
The process of extracting water from the Calcium Chloride brine requires a lot of energy, but fortunately burning biomass produces a lot of heat energy (and biomass is low cost or free). One great thing about this method is that no electricity is needed, and it represents a good way to make water from air without electricity. It can also be a good DIY project for students.
Trying to use the sun to boil the brine can also work but it needs to be concentrated solar energy using mirrors in order to produce a temperature sufficient to evaporate the brine. Ultimately you want the brine to boil, which results in fast water vapor production, and condensation. The key components used in this system are Calcium Chloride, biomass, and a condensation apparatus which can be built. All of these should be quite inexpensive to get for the average person. The key is to avoid special equipment/manufacturing requirements, just like for the production of cheap energy. Ultimately we want low-tech solutions. Calcium Chloride can be purchased easily and at low cost since it is a commonly used deicer in winter. Water-from-air systems with significant technological requirements (such as machines) are fine but their cost must be low and that is usually not the case unless they are mass produced.
The figures below illustrate the set up of this water-from-air system involving Calcium Chloride.
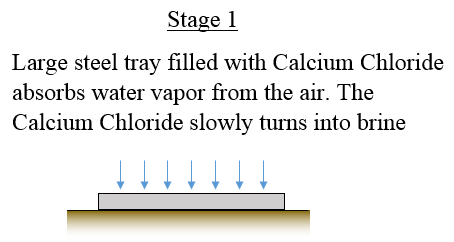
The large steel tray exposes a large surface area of Calcium Chloride to the air which means faster water absorption. Note that this tray should be made of stainless steel since it can resist the heat of the fire without corroding.
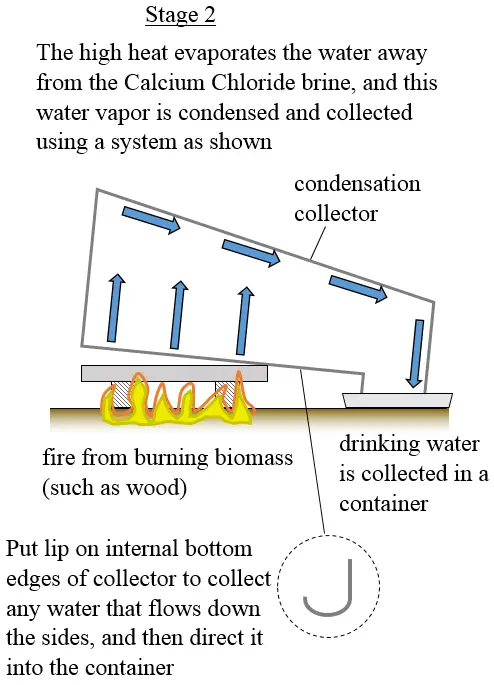
The large surface area of the tray means that you can put a large fire underneath it to extract water as quickly as possible.
The process stops when all the water chemically bonded to the Calcium Chloride is evaporated away. The collected water is then suitable for drinking, assuming of course the condensation collector and container are clean to begin with.
Lastly, locate this water-from-air system outdoors, far away from anything flammable. Always take extra precautions when working with fire!
Return to Engineering page
Return to Real World Physics Problems home page
Free Newsletter
Subscribe to my free newsletter below. In it I explore physics ideas that seem like science fiction but could become reality in the distant future. I develop these ideas with the help of AI. I will send it out a few times a month.