How Light Bulbs Work

Incandescent light bulb. Source: https://commons.wikimedia.org/wiki/File:Gluehbirne_2_db.jpg
There are two ways to produce visible light. The first way is by using the process of thermal radiation in which you heat up an object to a sufficiently hot temperature and it will give off visible light as a result. This is called incandescence. In this type of light production, the atoms and molecules of the heated object move rapidly (at the microscopic level) with random motion, and have kinetic energy as a result. The hotter the object, the faster the atoms and molecules move. These atoms and molecules consist of charged particles (i.e. protons and electrons). The interactions between these particles, during their motion, result in charge-acceleration and dipole-oscillation. This results in the electrodynamic generation of coupled electric and magnetic fields, which results in the emission of photons, which are the basic energy unit of electromagnetic radiation, such as visible light. For thermal radiation sources, the spectrum of electromagnetic radiation consists of the emission of photons over a range of frequencies. This is due to the random nature of the molecule and atomic motion over a range of energy levels. This in turn produces photon emission over a range of frequency levels (i.e. energy levels), with higher frequencies corresponding to higher energy levels. The average energy increases as the temperature of the object increases, with the various energies distributed about this average. In reality, all objects with a temperature above absolute zero emit electromagnetic radiation due to their temperature (which is known as thermal radiation). But it is only when the temperature of the object is high enough that we can see this radiation. The minimum temperature of an object necessary for the electromagnetic radiation emitted from it to be visible is about 500 degrees Celsius. At this temperature the object glows a dull red. The color red is the color corresponding to the lowest temperature in the visible spectrum, produced from thermal radiation. The color red has the lowest emission frequency of all the colors in the visible spectrum. This corresponds to the lowest emission energy of all the colors in the visible spectrum. For temperatures below this the electromagnetic radiation is not visible to the human eye. An infrared camera is necessary to detect electromagnetic radiation (i.e. thermal radiation) whose energy level falls below that of the visible spectrum. The picture below shows hot embers of a fire that are hot enough to glow red.

Source: https://commons.wikimedia.org/wiki/File:Embers_01.JPG. Author: Jens Buurgaard Nielsen
The production of visible light via thermal radiation is exploited in incandescent light bulbs. In these bulbs a tungsten filament inside them is heated to a temperature of about 2500 degrees Celsius, using an electric current. At this temperature a fraction of the electromagnetic radiation produced from the hot filament falls in the visible spectrum. However, the majority of the electromagnetic radiation is emitted in the invisible infrared part of the electromagnetic spectrum. This means that incandescent light bulbs are inefficient as a light source (in which a minority of the electromagnetic radiation is emitted in the visible part of the electromagnetic spectrum). If the filament of incandescent light bulbs could withstand hotter temperatures their efficiency would increase. To produce visible light most efficiently the temperature of the filament would have to be about 6300 degrees Celsius, which is hotter than the surface of the sun. At this temperature the maximum percentage of the electromagnetic radiation is emitted in the visible part of the electromagnetic spectrum. However, no known material can withstand a temperature of 6300 degrees Celsius without melting.
The second way to produce light is with luminescence, in which light is produced from an object without it being heated. This is caused by the absorption of energy by appropriate materials. This energy can come from radiation in the x-ray or ultraviolet region of the electromagnetic spectrum, electron beams, chemical reactions, and so on. This type of electromagnetic radiation (i.e. light) production is a quantum phenomenon in which the absorption of energy (such as from ultraviolet radiation) "excites" the outermost electrons surrounding the nuclei of the atoms of the absorption material. As a result these electrons "jump" to higher energy states. But this higher energy state is unstable so the electrons return to their original unexcited state, and release their absorbed energy as a result, in the form of photons (i.e. electromagnetic radiation). This excited state does not correlate with atomic and molecular motion (which is associated with the temperature of an object), as is the case with thermal radiation. This is why luminescence does not require an object to be heated in order to give off electromagnetic radiation (e.g. visible light). Luminescence only involves the electrons of atoms and not the atoms themselves.
The following are examples of the different types of luminescence:
• Chemiluminescence - is the emission of light as the result of a chemical reaction. The picture below shows an example of this type of light production.

Source: https://commons.wikimedia.org/wiki/File:Chemoluminescent_reaction.jpg. Author: https://en.wikipedia.org/wiki/User:Deglr6328
• Bioluminescence - is the emission of light as a result of a biochemical reaction inside a living organism. It is a form of chemiluminescence. Familiar examples of this are fireflies and glow worms, which emit bioluminescent light. The picture below shows a glow worm.

Source: https://commons.wikimedia.org/wiki/File:Lampyris_noctiluca.jpg
• Electrochemiluminescence - is the emission of light as a result of electrochemical reactions.
• Crystalloluminescence - is the emission of light as a result of crystallization.
• Electroluminescence - is the emission of light as a result of an electric current flowing through a substance. A light-emitting diode (LED) is a common light source utilizing the principle of electroluminescence.
• Cathodoluminescence - is the emission of light as a result of a luminescent material (such as a phosphor) being struck by electrons. A familiar example is the generation of light by an electron beam striking the phosphor-coated inner surface of a television screen that uses a cathode ray tube. This causes the emission of photons which have wavelengths in the visible spectrum, thereby creating a visible image on the television screen.
• Mechanoluminescence - is the emission of light as a result of a mechanical action on a solid, such as with ultrasonic sound waves, or other means.
• Photoluminescence - is the emission of light from matter upon absorption of photons (i.e. electromagnetic radiation). For example, in commonly used fluorescent lamps a phosphor coating on the inner lamp surface emits visible light upon absorbing electromagnetic radiation in the ultraviolet part of the electromagnetic spectrum. This ultraviolet radiation is emitted by mercury vapor inside the lamp that is excited by passing an electric current through it. A fluorescent lamp converts electrical energy into visible (useful) light much more efficiently than incandescent light bulbs.
• Radioluminescence - is the emission of light from a material upon bombarding it with ionizing radiation, such as beta particles.
• Thermoluminescence - is the emission of light resulting from the re-emission of absorbed energy when a substance is heated. This is a form of luminescence exhibited by certain crystalline materials, such as some minerals, in which previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light when the material is heated.
• Cryoluminescence - is the emission of light resulting when an object is cooled.
The visible range is a significant part of the electromagnetic radiation emission from the sun. The part of the electromagnetic spectrum of the sun within the visible range contains the strongest electromagnetic radiation emission of the sun, in terms of power output per range of wavelength (of the emitted electromagnetic radiation). As humans we evolved the ability to see electromagnetic radiation inside the visible range of the electromagnetic spectrum (that's why we call it the visible range). Naturally we want artificial light sources (incandescent or luminescent) to produce as much electromagnetic radiation as possible in this range.
The figure below shows the spectral distribution of different light sources over the range of visible wavelengths. The relative emission power is shown as a function of wavelength. This figure shows that there is greater emission power at some wavelengths than at other wavelengths. Note that incandescent lamps and sunlight (which is also an incandescent light source) produce a smooth and continuous electromagnetic spectrum over a range of wavelengths. This is due to the random nature of the molecule and atomic motion over a range of energy levels, for thermal radiation sources. Fluorescent lamps, however, produce a spectrum with a continuous curve with superimposed discrete bands. The continuous part of the spectrum curve results from the halophosphor and rare earth phosphor coating on the inner lamp surface. The discrete bands (or line spectra) results from the luminescent light emission from the mercury vapor inside the fluorescent lamp. These discrete bands correspond to discrete energy levels of the "excited" electrons of the mercury atoms. This figure illustrates that incandescent light sources, due to their continuous and relatively smooth spectral distribution, produce better color balance than fluorescent light sources.
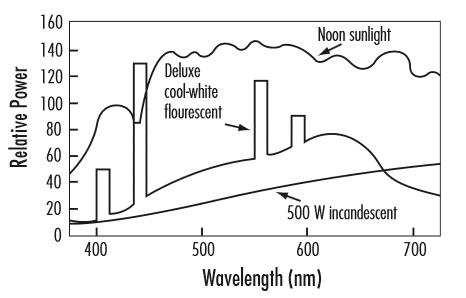
Reference: https://www.hydro.mb.ca/regulatory_affairs/electric/gra_08_09/information_requests/Appendix_54-LightingGuide.pdf
The figure below shows the electromagnetic spectrum over a large range of wavelengths, for objects at different temperatures (which emit thermal radiation). The 5000 K temperature is close to the surface temperature of the sun and therefore an object at this temperature emits a significant amount of electromagnetic radiation in the visible range. The 3000 K temperature is approximately that of an incandescent light bulb. Due to its lower temperature it emits proportionally less electromagnetic radiation energy in the visible range than an object at 5000 K, and is therefore less efficient as a (visible) light source. The dome-shaped curves shown in this figure are idealized in terms of their distribution (shape), but are reasonably accurate nonetheless. Objects which emit electromagnetic radiation with this distribution are referred to as black body emitters. The black curve represents the classical theory of Rayleigh-Jeans for an object at a temperature of 5000 K. For smaller wavelengths towards the ultraviolet this theory is very much in error.
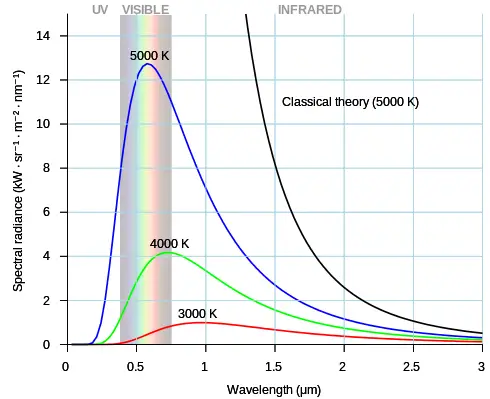
Source: https://commons.wikimedia.org/wiki/File:Black_body.svg. Author: https://commons.wikimedia.org/wiki/User:Darth_Kule
It should be noted that the topic covered here is with regards to light-emitting sources. Objects can be of a certain color due to the selective reflection of electromagnetic radiation from their surfaces. In this regard the color of the object corresponds to the portion of the electromagnetic spectrum that is visible in this color, and it is this part of the electromagnetic spectrum which is reflected from the object (and not emitted from it). The portion of the electromagnetic spectrum that is reflected off an object depends on the surface properties of the object. To test whether the color of an object is due to the emission of electromagnetic radiation of that color or the reflection of electromagnetic radiation of that color, remove all light sources in the area you are in. The objects that are emitting electromagnetic radiation will stay lit and all other objects will be dark.
Reference
http://www.britannica.com/science/luminescence
Return to Physics Essays page
Return to Real World Physics Problems home page
Free Newsletter
Subscribe to my free newsletter below. In it I explore physics ideas that seem like science fiction but could become reality in the distant future. I develop these ideas with the help of AI. I will send it out a few times a month.